Title:
Assessment of the Effects of Different Compositions of Ingredients Used
on the Characteristics of an Emulsion Formulation
Objective:
- To determine the effect of HLB surfactant on the emulsion stability.
- To study the effect on physical and stability of the emulsion when the different amount of emulsifying agent have been used.
Introduction:
Emulsion
is a 2 phase systems that thermodynamically not stable. It contains at least 2
immiscible liquid which internal phase distributed homogenously in the other
liquid phase (external phase). Emulsion can be classified into oil in water
emulsion (o/w) and water in oil emulsion (w/o). Emulsion is stabilizing using
the emulsifying agents. The emulsifying agent can be classified into 4 types,
which are hydrophilic colloid, fine solid phase, surface active agent and
surfactant.
The
HLB (hydrophilic-lipophilic balance) have been used to determine the quantity
and the type of surfactant need to be used to prepare a stable emulsion. Every
surfactant has it own HLB range which is from 1 (lipophilic) until 20
(hydrophilic). Normally, the usage of 2 emulsifying agent will form a very
stabilize emulsion preparation. The HLB value can be determine using the
equation below:
HLB
value =
|
[(quantity
of surfactant 1)(HLB of surfactant 1) + (quantity of surfactant 2)
(HLB
of surfactant 2)]
|
quantity of surfactant 1 + quantity of surfactant 2
|
Apparatus:
8 test tubes, measuring cylinder
50ml, pipette, droppers, weighing boat, mortar and pestle,
Light microscope, glass slide,
cover slip, beaker, centrifuge, Coulter Counter machine, Vortex mixture law, viscometer,
water bath (45 degree Celsius), refrigerator (4 degree Celsius).
Materials:
Palm oil, arachis oil, olive oil,
mineral oil, distilled water, span 20, tween 80, Sudan III solution (0.5%) and
ISOTON III solution.





Figure 1: Acacia gum

Figure 2: Span20

Figure 3: Tween 80

Figure 4: Palm Oil
Procedure:
1. 8
tests tube is labeled and 1cm from the bottom is marked at the tests tube.
2.
4mL of oil (refer to table I) and 4ml of distilled
water is mixed in the test tube.
Table I
Group
|
Type of oils
|
1,5
|
Palm oil
|
2,6
|
Arachis oil
|
3,7
|
Olive oil
|
4,8
|
Mineral oil
|
3. Span
20 and Tween 80 is added to the mixture according to the amount given in the
table below. The mixture is mixed using the Vortex mixing machine for about 45
seconds. The time taken for separation to occur until it reaches the 1cm marked
is recorded. The HLB value for each sample is determined.
The mixture is mixed using Vortex mixing machine:
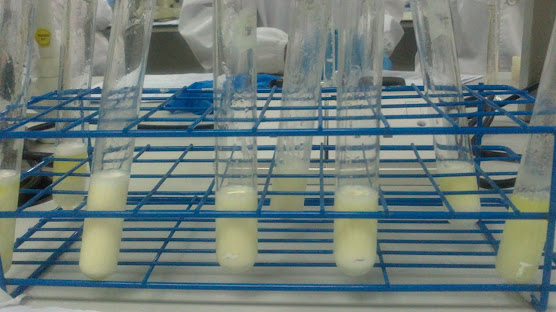
Figure 5: Emulsions with different composition of Span 20 and Tween 80
Table II
Tube no.
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
Span 20 (drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
Tween 80 (drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
4. The
Sudan III mixture is dropped into 1g of each of the emulsion produced in the
weighing boats. The color dispersion is described and compared with other
emulsion formulation. The emulsion is observed under the light microscope. The
structure and globule size are determined and drawn to compare with other
emulsion.



Figure 6: Sudan III solution is added to the emulsion

Figure 7: The emulsion is observed under light microscope
5. The Mineral Oil Emulsion (50g) is
prepared using wet gum method following the formulation below:
Mineral
oil
|
Referred
to table III
|
Acacia
|
6.25g
|
Syrup BP
|
5ml
|
Vanillin
|
2g
|
95% Ethanol
|
3ml
|
Distilled water
|
qs. 50ml
|
6. 40g
of the emulsion is placed in a 50ml beaker and homogenize for 2 minute using
homogenizer
7. 2g
of the sample before and after been homogenized is taken out and placed in the
weighing boats. Sudan III solution is dropped into the emulsion. The texture,
consistency, appearance of the oil and the color dispersion is determined and
compared which is it is observed under the light microscope
8. 15g
of the emulsion that have been homogenized is taken and the viscosity is
determined using the viscometer that has been calibrated using the “Spindle”
LV-4 type. The sample is placed at 45°C for about 30 minutes and at 4°C for 30
minutes afterward. The viscosity is determined afterward.
9. 5g
of the emulsion is centrifuged in 4500rpm, for 30 minutes at 25oC. The
separation is measured and the ratio is determined.
Results:
The structure and globule size under light microscope:

Figure 8a: The emulsion in tube 1

Figure 8b: The emulsion in tube 2

Figure 8c: The emulsion in tube 3

Figure 8d: The emulsion in tube 4

Figure 8e: The emulsion in tube 5

Figure 8f: The emulsion in tube 6

Figure 8g: The emulsion in tube 7
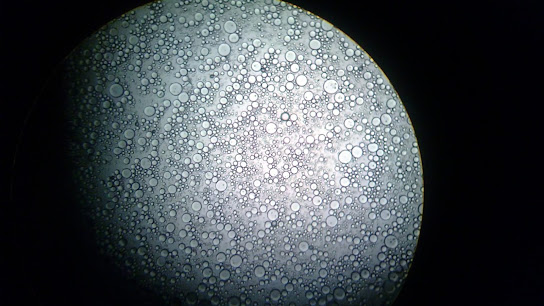
Figure 8h: The emulsion in tube 8
Phase Separation and HLB Value:
Tube no.
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
|
Span 20 (drops)
|
15
|
12
|
12
|
6
|
6
|
3
|
0
|
0
|
|
Tween 80 (drops)
|
3
|
6
|
9
|
9
|
15
|
18
|
15
|
0
|
|
HLB Value
|
9.67
|
10.73
|
11.34
|
12.44
|
13.17
|
14.09
|
15.00
|
0
|
|
Time taken for Phase Separation (min)
|
Palm Oil
|
38
|
127
|
123
|
79
|
121
|
126
|
11
|
1
|
Arachis Oil
|
>120
|
>120
|
>120
|
45
|
37
|
18
|
77
|
2
|
|
Olive Oil
|
40
|
>120
|
>120
|
64
|
59
|
21
|
34
|
5
|
|
Mineral Oil
|
>90
|
>90
|
>90
|
>90
|
55
|
47
|
12
|
2
|
|
Stability
|
Palm Oil
|
/
|
/
|
/
|
/
|
/
|
/
|
X
|
X
|
Arachis Oil
|
/
|
/
|
/
|
X
|
X
|
X
|
X
|
X
|
|
Olive Oil
|
X
|
/
|
/
|
X
|
X
|
X
|
X
|
X
|
|
Mineral Oil
|
/
|
/
|
/
|
/
|
X
|
X
|
X
|
X
|
|
Viscosity of emulsion :
Difference
= (Average of after temperature cycle-Average of before temperature cycle )/(Average+SD of before temperature cycle)×100%
At 20 mL of
Mineral Oil :
Readings
|
Group
|
Viscosity
(cP)
|
Average + SD
|
|||
1
|
2
|
3
|
||||
Before temperature cycle
|
1
|
150
|
100
|
150
|
78.33 + 57.57
|
|
5
|
20
|
20
|
30
|
|||
After temperature cycle
|
1
|
200
|
200
|
250
|
114.17+103.86
|
|
5
|
10
|
10
|
15
|
|||
Difference (%)
|
45.76%
|
|||||
At 25 mL of Mineral Oil :
Readings
|
Group
|
Viscosity
(cP)
|
Average + SD
|
|||
1
|
2
|
3
|
||||
Before temperature cycle
|
2
|
30
|
60
|
120
|
60+28.28
|
|
6
|
50
|
50
|
50
|
|||
After temperature cycle
|
2
|
120
|
120
|
90
|
105+11.18
|
|
6
|
100
|
100
|
100
|
|||
Difference (%)
|
75%
|
|||||
At 30 mL of
Mineral Oil :
Readings
|
Group
|
Viscosity
(cP)
|
Average + SD
|
|||
1
|
2
|
3
|
||||
Before temperature cycle
|
3
|
420
|
480
|
540
|
490+46.19
|
|
7
|
450
|
500
|
550
|
|||
After temperature cycle
|
3
|
2800
|
2650
|
2460
|
2626.67+219.97
|
|
7
|
3000
|
2500
|
2350
|
|||
Difference (%)
|
436.06%
|
|||||
For 35 mL of
Mineral Oil :
Readings
|
Group
|
Viscosity
(cP)
|
Average + SD
|
|||
1
|
2
|
3
|
||||
Before temperature cycle
|
4
|
5750
|
5800
|
5850
|
7350+1563.83
|
|
8
|
8900
|
8900
|
8980
|
|||
After temperature cycle
|
4
|
7300
|
7100
|
7200
|
11325+4129.14
|
|
8
|
15650
|
15600
|
15100
|
|||
Difference (%)
|
53.80%
|
|||||
Average viscosity before and after temperature cycle
for different amount of mineral oil
Amount of Mineral Oil (mL)
|
Average Viscosity (cP) (x ± SD)
|
Viscosity difference (%)
|
|
Before temperature cycle
|
After temperature cycle
|
||
20
|
78.33 +
57.57
|
114.17+103.86
|
45.76% ±80.41
|
25
|
60+28.28
|
105+11.18
|
75% ±60.47
|
30
|
490+46.19
|
2176.67+500.89
|
436.06% ±376.23
|
35
|
7350+1550.27
|
11325+4129.14
|
53.80% ±164.04
|
Separation height
Height ratio = (Separation phase )/(Emulsion phase)
Height(mm)
|
|
Seperation Phase
|
29
|
Original Emulsion
|
46
|
Height Ratio
|
29/46 = 0.63
|
Height (mm)
|
||||||||
Group
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
Separation
Phase
|
29
|
35
|
14
|
15
|
40
|
35
|
11
|
11
|
Original
emulsion
|
46
|
55
|
50
|
55
|
50
|
50
|
50
|
50
|
Height
ratio
|
0.63
|
0.64
|
0.28
|
0.27
|
0.8
|
0.70
|
0.22
|
0.22
|
Group 1 and 5 (20ml):-
Group 2 and 4 (25ml):-
Average: 0.715 Average:
0.670
Standard Deviation: 0.120 Standard Deviation: 0.042
Group 3 and 7 (30ml):-
Group 4 and 8 (35ml):-
Average: 0.250 Average: 0.245
Standard Deviation: 0.042 Standard Deviation: 0.035
Mineral oil (ml)
|
Separation phase ratio (x ± SD)
|
20
|
0.715 ± 0.120
|
25
|
0.670 ± 0.042
|
30
|
0.250 ± 0.042
|
35
|
0.245 ± 0.035
|
Discussion:
1) What are the HLB
values that will produce a stable emulsion? Discuss.
HLB System enables us to assign a number to the ingredient or
combination of ingredients we want to emulsify, and then to choose an
emulsifier or blend of emulsifiers having this same number. The HLB value for
the combination of emulsifying agent can be calculated by using this formula:


HLB range
|
Use
|
0-3
|
Anti foaming agents
|
3-6
|
W/O emulsifying agents
|
7-9
|
Wetting and spreading
agents
|
8-18
|
O/W emulsifying agents
|
15-20
|
Solubilisers.
|
The table above contains the range
of the HLB value with the suitable uses. The required HLB for a mixture of
oils, fats and waxes present in a formulation can be calculated theoretically
form the proportion of each component in the oil phase. Usually it is better to
have a mixture of emulsifiers such as oil soluble (low HLB) and a water soluble
(high HLB) surfactant.
2) Compare the physical features of mineral
oil emulsion and give explanation. What is Sudan III Test? Compare dispersion
of color in the emulsion formed and give explanation.
Figure 9: Emulsion added with Sudan III
Before
homogenization, the globules size in the emulsion is not uniform. There are
some big globules and small globules in the emulsion. The size and shape of the
globules is not consistent. Some of the oil globules which are red in color
have bigger circular shape while others are irregular shape red globules.
Besides that the globules are distributed unevenly throughout the emulsion. The
oil globules are unstable with a small size with a large surface area. The
larger the surface area, the larger the surface energy. In order to reduce the
surface energy and to stabilize the emulsion, the small globules tend to
coalesce to become large globules. The emulsion is non homogenous and phase
separation may occur. The oil degree of the emulsion before homogenization is
high and more greasy. The color of the emulsion is yellowish.
After
homogenization, the globules are broken down into smaller one. The globule size
is also more uniform than the emulsion before homogenization. The oil globules
are distributed more evenly. The size and shape of the oil globules are more
consistent. The red color oil globules are circular in shape.
The emulsion is more homogenous and less greasy. The emulsifier is evenly
adsorbed on the interfacial surface of the globules to promote stable emulsion.
Homogenization process is to break down the crystal clump and make the oil
globules more stable in aqueous phase. The homogenized emulsions are white in
color. As the oil globules are evenly distributed, the drugs that are dispersed
inside the oil phase will allow accurate dose dispensed to the patients.
3) Plot and give comments on:
a)
Graph of the sample’s viscosity before and after the temperature cycle against
the variety contents of mineral oil.
Amount of Mineral Oil (mL)
|
Average Viscosity (cP) (x ± SD)
|
Viscosity difference (%)
|
|
Before temperature cycle
|
After temperature cycle
|
||
20
|
78.33 +
57.57
|
45.76% ±80.41
|
|
25
|
60+28.28
|
105+11.18
|
75% ±60.47
|
30
|
490+46.19
|
2176.67+500.89
|
436.06% ±376.23
|
35
|
7350+1550.27
|
11325+4129.14
|
53.80% ±164.04
|

From the graph
above which is obtained based on the results of our experiment, the amount of
oil used influence the viscosity of the emulsion. Besides that the temperature
which the emulsion is stored also has effects on the viscosity of emulsion. The
acacia act as an emulsifying agent which will contribute the viscosity of the
emulsion. But, in this experiment, the amount of acacia used for each group is
the same. Thus, the amount of acacia added will not be studied in this
experiment.
For every
different amount of oil used, the viscosity after the temperature cycle
increased. The emulsion was put in water bath at 450C for 30 minutes
and then freezed at 40C for 30 minutes. Theoretically, the viscosity
of the emulsion after being immersed in the water bath will decrease. This is
because the thermal energy of the droplets and the emulsifying agent at oil in
water interface increased when temperature increase. As temperature increased,
the droplets collide with each other more frequently, interfacial viscosity
decreases and thus the droplets will coalescence more rapidly. In addition, the
solubility of the emulsifying agent increased when temperature increased, the
emulsion is unstable and the viscosity decreased.
The viscosity of
the emulsion increased after the emulsion was freezed. When the temperature
decreased, the kinetic energy of the droplet decreased and the droplets collide
with each other less frequently. Thus, the viscosity of the emulsion increased.

From this graph,
we know that the viscosity difference increases from 20mL of oil to 30mL of
oil. The viscosity increases with the increase of the amount of oil. From 30mL
of oil to 35mL of oil, the viscosity difference decreases. This is because the
emulsion already possesses the highest viscosity in the increase of the amount
of oil.
From the result
obtained from the experiment, the viscosity of the emulsion with 25mL of oil
has the lowest viscosity which is higher than emulsion with 20mL of oil. The
viscosity of emulsion should increase with the increase of the amount of oil
before the amount of dispersed phase exceeds the continuous phase which may
result in phase inversion.
Thus, there may be
some error occurred during the experiment. Error may occur during the
preparation of emulsion. Wet gum method carried out may be failed to get a good
emulsion. Trituration during preparation may not be applied properly. There is
also phase separation of emulsion during the viscosity test which may affect
the reading of the viscometer. Some groups prepared the primary emulsion but
other did not. There are different standard between each groups.
4) Plot a graph of separated phase ratio
formed from the centrifugation process versus the amount of mineral oil used.
Explain.
Mineral oil (ml)
|
Separation phase ratio (x ± SD)
|
20
|
0.715 ± 0.120
|
25
|
0.670 ± 0.042
|
30
|
0.250 ± 0.042
|
35
|
0.245 ± 0.035
|

Phase separation ratio
is used to indicate stability of an emulsion. A high ratio of phase separation
represents an unstable emulsion where two separated phases can be observed. Due
to unstable emulsion, uniformity of drug in the emulsion will be greatly altered
and the accuracy of dose being administered into patient might affected.
Based on the graph plotted, separated phase
ratio decreases from 20mL palm oil emulsion to 25mL arachis oil emulsion,
decreases from 25mL arachis oil emulsion to 30mL olive oil emulsion and
decreases also from 30mL olive oil emulsion to 35mL mineral oil emulsion.
According to theory, the
separation phase ratio should increase with the increasing of the mineral oil
contained in the formulation. This
is because the added amount of oil in emulsion has exceeded the oil amount at
which a stable emulsion can be formed.
Phase separation will occur at a faster rate.
However, the results we obtained from the
experiment do not follow this theory. This may be due to several errors that
occurred during experiment. This may due to some experimental errors
such as measurement mistakes and systemic error. The experimental errors
include inaccuracy in
measuring the amount of oil before forming the emulsion, insufficient
homogenisation that has been carried out on emulsion and the height of
separated phase is not being measured accurately.
Thus, an
appropriate amount of oil should be used in the emulsion to prevent phase
separation as the amount of oil affects the uniformity and stability of the
dose of drug administered. The drug particles should be dispersed in the
emulsion uniformly in an ideal emulsion.
5) What is the function of each material used in the preparation of
this emulsion? How the usage of the different materials content will affect the
physical properties and stability of an emulsion formulation?
Palm oil, arachis oil, olive oil
and mineral oil act as oily phase in the emulsion. It is either dispersed phase
or continuous phase depends on the type of emulsion formed in which it is the
dispersed phase in o/w emulsion and vice versa. In this case, the
mineral oil used in the formulation acts as the dispersed phase while the
distilled water is the continuous phase. Thus, this is a oil in water (o/w)
emulsion. Acacia is added in this formulation which act as emulsifying agent in
order to reduce the surface tension at the interphase surface. Thus, the
mineral oil globules can disperse uniformly in the continuous phase and any
instability of emulsion such as flocculation and creaming or breaking or
coalescence won’t occur. Other instabilities of emulsion include Ostwald
ripening, phase inversion and any miscellaneous physicochemical changes such as
appearance, odour and colour.

As for your
information, emulsifying agents have to be added in the correct amount based on
HLB value. The amount of the acacia added will determine the uniformity of oily droplets
dispersed throughout the emulsion.. If the acacia that added is in adequate
amount, then the oily phase will disperse uniformly in the continuous phase.
However, if either too many or too less acacia is added, the stability of the
emulsion will greatly decrease. Besides maintaining separation of droplets in
disperse phase, it also increases the viscosity of the emulsion formed. As acacia is a natural
polysaccharides and susceptible to microbial attack, thus alcohol is added into
emulsion as preservative to prevent microbial growth. Physicochemical
properties can be enhanced with the addition of alcohol. However, only appropriate amount of alcohol
can be added into emulsion to give greatest antimicrobial effect and prevent
toxicity.
Besides, syrup and
vanillin which act as the sweetener and flavouring respectively are added in
this formulation. Syrup also can increase the viscosity of the emulsion formed.
Amount of turpentine oil
(dispersed phase) and distilled water (continuous phase) added to form an
emulsion is important in determining the type and stability of emulsion formed.
The volume of dispersed phase should not be more than the volume of the
continuous phase. The stability of emulsion formed will decrease if the volume
of dispersed phase exceeds 50% of the emulsion. Phase inversion tends to occur
for emulsions containing more than about 70% dispersed phase.
Conclusion:
Low HLB surfactant
value, 3-9 is suitable to be used in water in oil emulsion. While, the high
value of HLB surfactant, 8-18 is suitable for the formation of oil in water
emulsion. Combination of surfactants, such as Span and Tween will form a more
stable emulsion than a single surfactant. Different oily phase need a different
value of HLB surfactant so that the most stable emulsion can be formed. The physical and stability of the
emulsions are different if the amount of emulsifying agent used is different.
References :
- Pharmaceutical
Practice, A.J. Winfield, J.A. Rees, I.Smith, 4th edition
- Composition
And Properties Of Drilling And Completion Fluid, R.Caenn, G.R. Grey,
H.C.H. Darley, 6th edition


No comments:
Post a Comment